Medical Translation Requirements: Complete Guide to Compliance
- AD VERBUM

- Nov 26, 2025
- 6 min read

Precision in medical translation saves lives. Every year, medical errors linked to language misunderstandings risk patient safety, with over 30 percent of translation mistakes in clinical settings leading to negative health outcomes. For many healthcare companies, meeting strict translation demands is not just about legal compliance—it directly impacts global patient care and corporate reputation. This guide breaks down what sets top-tier medical translation apart, how experts ensure accuracy, and why combining human expertise with advanced technology is now the benchmark in the field.
Table of Contents
Key Takeaways
Point | Details |
Medical Translation Requirements | Translators must have advanced educational backgrounds, specialized medical knowledge, and linguistic mastery to ensure precision and compliance. |
Regulatory Standards | Key standards like ISO 17100 and HIPAA ensure safety and accuracy in medical translation across international markets. |
Quality Control Processes | A robust multilevel review process involving subject matter experts is essential to guarantee translation quality and regulatory adherence. |
Data Security Strategies | Implementing secure infrastructures and strict terminology management is crucial for maintaining data integrity and compliance in medical translation projects. |
Defining Medical Translation Requirements
Medical translation is a specialized discipline that demands exceptional linguistic precision, subject matter expertise, and rigorous compliance standards. According to imiaweb, translators in this field must possess native or near-native language proficiency coupled with advanced analytical skills and comprehensive cultural understanding.
The core requirements for medical translation professionals encompass several critical dimensions:
Advanced Educational Background: Formal college-level training in translation theory and practice
Specialized Medical Knowledge: Expert understanding of complex medical terminology
Linguistic Mastery: Profound comprehension of both source and target language nuances
Research Competence: Ability to utilize specialized medical dictionaries and conduct extensive terminology research
Professional medical translators must navigate an intricate landscape of linguistic challenges. imiaweb emphasizes that translators cannot merely translate words, but must thoroughly understand the source text’s contextual and technical implications. This requires deep subject matter expertise, particularly when addressing languages with significant regional variations.
Beyond linguistic skills, medical translation demands an unwavering commitment to accuracy. Precision is paramount. A single mistranslated medical term could potentially compromise patient safety, research integrity, or regulatory compliance. Professional translators must therefore combine linguistic excellence with meticulous attention to technical details, ensuring that every translated document maintains the original’s exact scientific meaning.
With the AI+HUMAN approach utilized by AD VERBUM, companies can leverage advanced technological tools while maintaining the critical human expertise necessary for nuanced medical translations. Our guide on specialized translation workflows provides additional insights into how cutting-edge translation technologies can enhance accuracy and efficiency in high-stakes medical documentation.
Key Regulatory Standards Worldwide
In the complex world of medical translation, regulatory standards serve as critical frameworks that ensure accuracy, safety, and compliance across international healthcare and medical device industries. Wikipedia highlights that ISO 17100:2015 provides comprehensive guidelines covering all aspects of the translation process, specifically targeting the quality and delivery of translation services.
Key international regulatory standards for medical translation encompass multiple critical domains:
ISO 17100: Translation service quality management
ISO 13485: Medical device risk management
HIPAA: Healthcare information privacy and security
GDPR: Data protection and personal information handling
Medical Device Regulation (MDR): Medical device safety and performance standards
Wikipedia emphasizes that ISO 13485 represents a critical international standard specifically designed for medical device risk management. This standard establishes a comprehensive framework for risk analysis, evaluation, control, and ongoing review, ensuring medical devices meet stringent safety requirements throughout their entire lifecycle.
Companies operating in global medical markets must meticulously navigate these complex regulatory landscapes. The AI+HUMAN approach utilized by AD VERBUM provides a robust solution, integrating advanced technological capabilities with human expertise to meet these demanding international standards. Our guide on specialized translation workflows offers deeper insights into how cutting-edge translation technologies can effectively address these intricate regulatory requirements.
Types of Medical Documents Translated
Medical translation encompasses a complex landscape of documentation critical to healthcare, research, and regulatory compliance. Wikipedia highlights that medical translation involves a comprehensive range of documents essential for multiple professional domains, including healthcare providers, medical device manufacturers, and pharmaceutical companies.
The primary categories of medical documents requiring precise translation include:
Clinical Documentation
Clinical trial reports
Patient medical records
Research study protocols
Regulatory Submissions
Drug data sheets
Medical device specifications
Compliance documentation
Patient-Facing Materials
Consent forms
Patient education materials
Informational brochures
imiaweb emphasizes that certain documents demand priority in translation, particularly those directly impacting patient understanding and safety. These include admission forms, procedural guidelines, privacy documentation, and critical release instructions that ensure patients comprehend medical processes and their rights.
The translation process extends beyond mere linguistic conversion. It requires a meticulous approach that preserves medical terminology, maintains regulatory compliance, and ensures cultural sensitivity. With the AI+HUMAN approach utilized by AD VERBUM, companies can navigate these complex translation requirements efficiently. For deeper insights into specialized translation workflows, check out our guide on AI+human translation.
Critical Quality Control and SME Roles
Quality control in medical translation represents a multifaceted and intricate process that goes far beyond simple linguistic conversion. imiaweb emphasizes that effective medical translation requires a comprehensive approach involving multiple rigorous verification stages to ensure absolute precision and reliability.
The quality control workflow typically involves several critical steps:
Initial Translation
Performed by qualified professional translators
Focuses on accurate initial content transfer
Multilevel Review Process
Secondary linguistic review
Technical accuracy verification
Contextual appropriateness assessment
Subject Matter Expert (SME) Validation
Specialized professional review
Terminology and technical precision check
Regulatory compliance confirmation
imiaweb underscores the pivotal role of Subject Matter Experts (SMEs) in medical translation quality control. These specialized professionals bring deep domain expertise, ensuring that translations not only communicate linguistically but also capture the precise technical nuances critical in medical documentation.
The AI+HUMAN approach utilized by AD VERBUM integrates advanced technological capabilities with human expertise, creating a robust quality control framework. By combining machine translation efficiency with expert human oversight, we guarantee translations that meet the most stringent accuracy and compliance standards. For a deeper understanding of our specialized translation methodologies, explore our guide on AI+human translation.
Data Security and Terminology Enforcement in Practice
Data security in medical translation represents a critical frontier of technological and procedural innovation. Wikipedia highlights that robust translation projects must integrate comprehensive data protection mechanisms, particularly when handling sensitive client information across complex linguistic landscapes.
Key strategies for maintaining data security and terminology precision include:
Secure Infrastructure
Proprietary, closed-loop translation environments
EU server-based operations
Zero public cloud data exposure
Terminology Management
Strict glossary enforcement
Contextual translation validation
Machine learning-assisted consistency checks
Compliance Frameworks
GDPR compliance
HIPAA information protection
ISO 17100 data handling standards
ArXiv introduces the groundbreaking METRIC-framework, which provides a systematic approach to assessing data quality in medical translation. This framework comprises 15 critical awareness dimensions designed to reduce potential biases, enhance robustness, and ensure precise terminology enforcement across complex translation projects.
The AI+HUMAN approach utilized by AD VERBUM transforms these theoretical frameworks into practical solutions. By combining advanced technological capabilities with human expertise, we create a translation ecosystem that guarantees unparalleled data security and linguistic precision. For a comprehensive exploration of our specialized translation methodologies, explore our guide on AI+human translation.
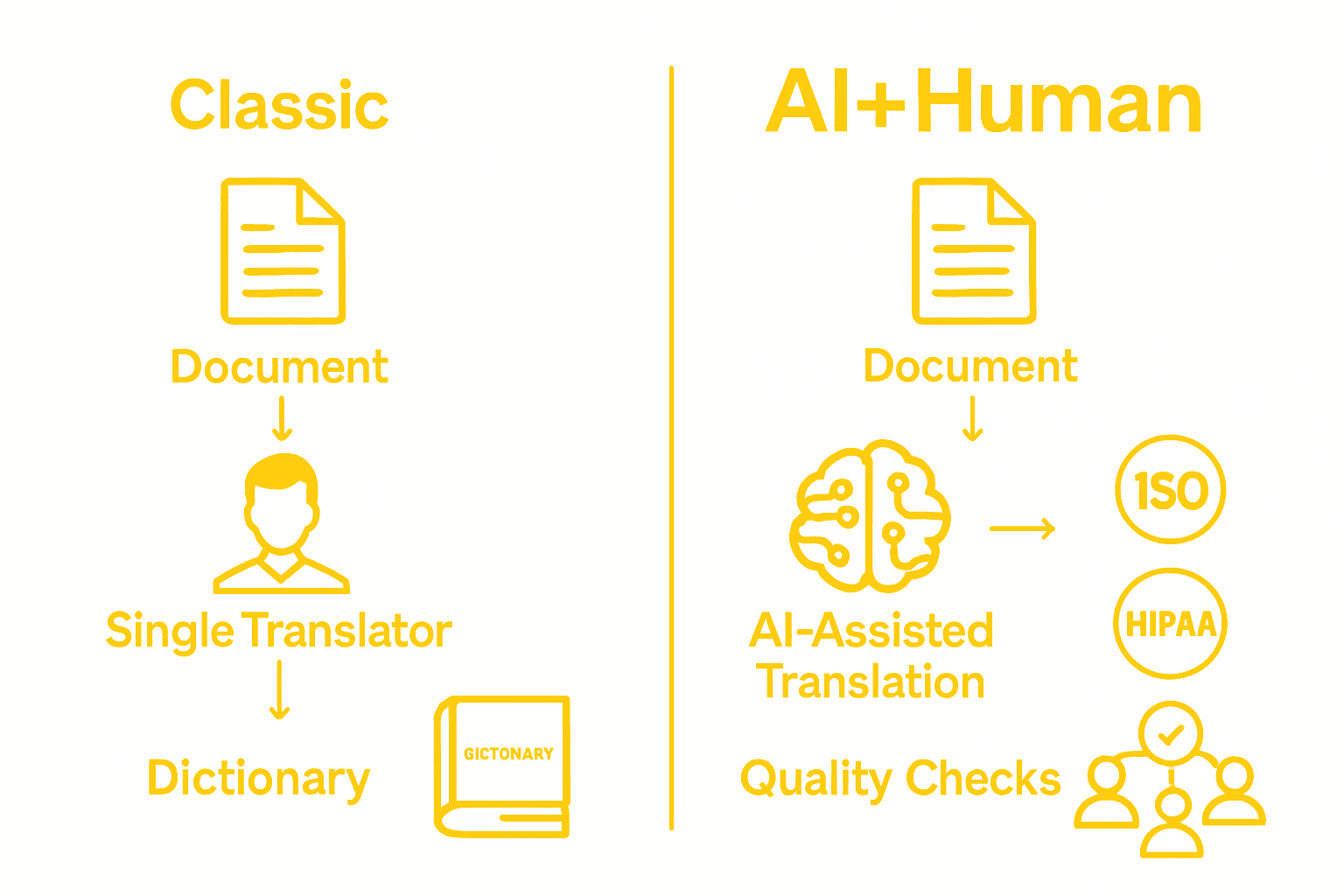
Ensuring Absolute Compliance and Precision in Medical Translation
The article “Medical Translation Requirements Complete Guide to Compliance” highlights the crucial need for accuracy, data security, and strict adherence to regulatory standards like ISO 17100 and HIPAA. Companies face steep challenges navigating complex medical terminology and safeguarding sensitive patient data while meeting international compliance mandates. The risks of mistranslation or data exposure are simply too great to allow conventional machine translation or public platforms.
AD VERBUM addresses these critical concerns with its proprietary AI+HUMAN workflow. Our private, EU-hosted Large Language Model ensures zero data leakage and enforces exact terminology governance, providing unmatched precision for high-stakes medical documents. Supported by a network of 3,500+ subject-matter expert linguists, AD VERBUM guarantees compliance with MDR, GDPR, and ISO standards through rigorous SME validation and tailored quality assurance.
Discover how to transform compliance challenges into reliable translation outcomes by exploring our specialized AI translation process in detail at AD VERBUM. For insights on combining advanced technology with human expertise, visit our guide on AI+human translation and learn about our specialized translation workflows. Take action now to ensure your medical translations meet the highest standards and avoid costly errors.
Frequently Asked Questions
What are the key qualifications for medical translators?
Medical translators should possess advanced educational backgrounds in translation theory, specialized medical knowledge, linguistic mastery in both source and target languages, and strong research skills for medical terminology.
Why is accuracy crucial in medical translation?
Accuracy is paramount in medical translation because a single mistranslation can compromise patient safety, research integrity, or regulatory compliance. Ensuring precise terminology and context is essential for maintaining the scientific meaning of medical documents.
What types of documents require medical translation?
Medical translation covers a wide range of documents, including clinical documentation (like trial reports and medical records), regulatory submissions (like drug data sheets), and patient-facing materials (like consent forms and educational brochures).
How does the AI+HUMAN approach enhance medical translation?
The AI+HUMAN approach combines advanced technological tools with human expertise, allowing for enhanced accuracy and efficiency in medical translations. This integration helps ensure compliance with international regulatory standards and maintains high quality in linguistic conversion.
Recommended



